TARRYTOWN, N.Y., June 17, 2025 (GLOBE NEWSWIRE) — Medtech Products Inc., a Prestige Consumer Healthcare Inc. company (“Medtech” or “Company”), is voluntarily recalling five lots of Little Remedies® Honey Cough Syrup (the “Product”) due to the presence of Bacillus cereus and loss of shelf-stability. Bacillus cereus (B. cereus) can cause two types of food-borne illnesses. One type is characterized by nausea, vomiting, and stomach cramps that can start 1 to 6 hours after eating or drinking contaminated food. The second type can cause stomach cramps and diarrhea that can start 8 to 16 hours after eating or drinking contaminated food. Diarrhea may be a small volume or profuse and watery. Although healthy individuals may suffer only short-term illness, exposure to high levels of foodborne B. cereus can cause death.
The affected lots were distributed nationwide in the United States through retailers and online from 12/14/2022 through 06/04/2025.
The table below identifies the UPC, lot numbers, and expiration dates of the Little Remedies® Honey Cough Syrup impacted by this recall.
| Item UPC | Lot # | Exp. Date |
| 7-56184-10737-9 | 0039 | 11/2025 |
| 0545 | 01/2026 | |
| 0640 | 02/2026 | |
| 0450 | 05/2026 | |
| 1198 | 12/2026 |
Little Remedies® Honey Cough Syrup is packaged in a 4 FL OZ (118 mL) amber bottle and is sold in an outer carton with the Lot Code appearing both on the bottle label and on the bottom of the carton (images below).
This recall does not include any other Little Remedies® products.
Bottle Label:
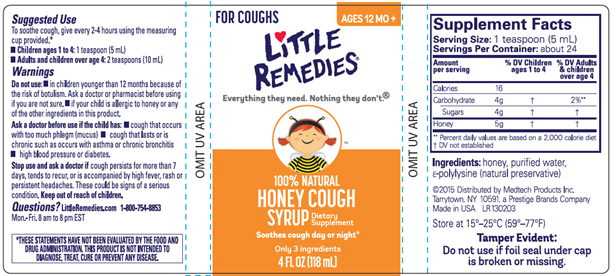
Carton:

No serious adverse events have been reported to date.
All lots of Little Remedies® Honey Cough 4 FL OZ (118 mL) still within expiry are being included in the scope of the recall.
Consumers who have the recalled Product should stop using it immediately and should contact their physician or healthcare provider if they have experienced any problems that may be related to the use of this Product. The company will also offer reimbursement for consumers who have purchased Products from the recalled lots.
Consumers with refund requests or questions regarding this recall can contact Medtech via e-mail at [email protected], through its website at https://www.prestigebrands.com/contact, or by phone at (800) 754-8853 on Monday – Friday 8:30-5:30 eastern time.
Adverse reactions or quality problems experienced with the use of this product may be reported to the FDA’s MedWatch Adverse Event Reporting program either online, by regular mail or by fax.
- Complete and submit the report Online: www.fda.gov/medwatch/report.htm
- Regular Mail or Fax: Download form www.fda.gov/MedWatch/getforms.htm or call 1-800-332-1088 to request a reporting form, then complete and return to the address on the pre-addressed form, or submit by fax to 1-800-FDA-0178.
This recall is being conducted with the knowledge of the U.S. Food and Drug Administration.
Photos accompanying this announcement are available at
https://www.globenewswire.com/NewsRoom/AttachmentNg/ca32fb6c-5efc-4785-bf61-239d42d91bfb
https://www.globenewswire.com/NewsRoom/AttachmentNg/fca77e04-87d0-45ef-b10e-7b13ae6e4adc




