– Oral semaglutide administered via the RaniPill® HC (RT-116) demonstrated comparable bioavailability, pharmacokinetics and weight loss to subcutaneous administration –
– RT-116 was well tolerated with no serious adverse events –
– Data adds to growing body of evidence of the RaniPill® platform’s potential to enable oral delivery of multiple obesity treatments –
– Phase 1 study for RT-114, an oral GLP-1/GLP-2 dual agonist for the treatment of obesity, expected to initiate in 2025 –
SAN JOSE, Calif., Feb. 05, 2025 (GLOBE NEWSWIRE) — Rani Therapeutics Holdings, Inc. (“Rani Therapeutics” or “Rani”) (Nasdaq: RANI), a clinical-stage biotherapeutics company focused on the oral delivery of biologics and drugs, today announced new pharmacokinetic and pharmacodynamic data from a preclinical study evaluating the oral delivery of the glucagon-like peptide-1 receptor (GLP-1) agonist semaglutide administered via the RaniPill® capsule.
Semaglutide is a GLP-1 receptor agonist that selectively binds to and activates the GLP-1 receptor mimicking its activity. GLP-1 is an incretin hormone and enterogastrone that stimulates insulin secretion, inhibits glucagon secretion, delays gastric emptying and reduces the production of stomach acid serving as a physiological regulator of appetite and food intake.
“We believe these data provide further validation of the RaniPill® oral delivery platform across a wide variety of injectable obesity treatments. We are encouraged to see that delivery of semaglutide via the RaniPill® capsule demonstrated comparable bioavailability, pharmacokinetics and weight loss to subcutaneous administration of semaglutide at the same dose,” said Talat Imran, Chief Executive Officer of Rani Therapeutics. “The target product profile of semaglutide in the RaniPill® capsule would be once-weekly oral administration of semaglutide therapy, which we believe may be more convenient for patients and could lead to improved adherence. Overall, the totality of the preclinical data we have generated in the obesity space demonstrating the successful delivery of both single and triagonist incretin therapies gives us confidence to continue building our obesity portfolio to unlock the true value of the RaniPill® technology. Looking ahead, we are excited to begin our Phase 1 study for RT-114, Rani’s GLP-1/GLP-2 dual agonist in partnership with ProGen this year.”
Currently, semaglutide is only available as a subcutaneous injection (SC) for the treatment of obesity and is marketed in the U.S. by Novo Nordisk as WEGOVY®. Worldwide sales for WEGOVY® were approximately $3.1 billion in the first half of 2024.
“There are currently no approved orally-administered GLP-1 agonists on the market for the treatment of obesity,” said Jesper Høiland, Senior Strategic Advisor for Rani Therapeutics and former executive at Novo Nordisk. “While RYBELSUS® is an oral version of semaglutide approved to improve glycemic control in adults with type 2 diabetes mellitus, it requires daily administration at a significantly higher dose than the subcutaneous equivalent. In comparison, the RaniPill HC harnesses advanced technology to deliver the same dose as the subcutaneous injection of semaglutide with expected weekly oral administration. I believe that a convenient, once-weekly oral version of semaglutide delivered via the RaniPill HC has the potential to be transformational as a next generation treatment that would impact how obesity is treated worldwide.”
Study Design
- The nonclinical pharmacokinetic (PK) and pharmacodynamic (PD) study was conducted to examine the effects of semaglutide delivered orally via the RaniPill (RT-116) versus a subcutaneous (SC) injection comparator group.
- The 2×2 crossover study was conducted in two parts with a period of 4 weeks separating the two groups to evaluate the PK, PD and safety of RT-116.
- Eight canines were randomized into two groups who received 0.5mg of semaglutide delivered via oral administration of the RaniPill® HC (N=4) or subcutaneous injection (N=4).
- Endpoints included plasma drug concentrations, body weight, food intake, lipid profile, and safety.
Data Highlights
- Semaglutide was successfully delivered in 7 of 8 canines that received the RaniPill® capsule.
- Semaglutide administered via the RaniPill® capsule was well tolerated with no serious adverse events.
- Cmax, Tmax, and AUC were comparable for semglutide administered via RaniPill® capsule and subcutaneous administration of semaglutide.
- Oral administration via the RaniPill® capsule demonstrated bioavailability and biological activity comparable to subcutaneous administration.
- The relative bioavailability of oral semaglutide was 107% versus subcutaneous administration.
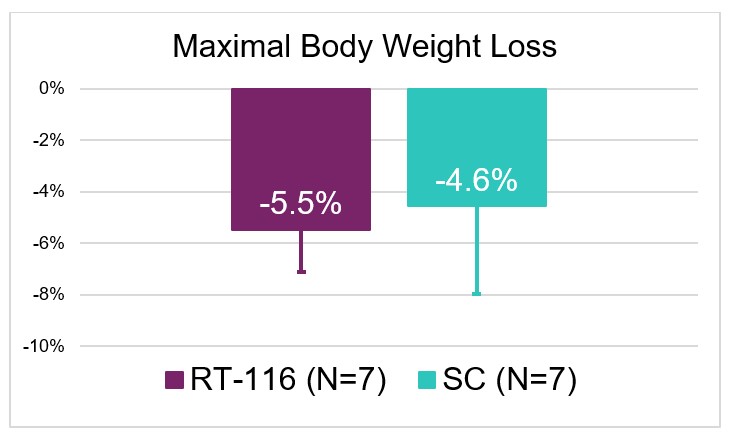
- Both groups saw comparable weight loss which appeared to be driven by decreased food intake that coincided with rises in plasma drug levels thus indicating there is a PD effect to treatment.
- Both groups saw comparable moderate decreases in serum triglycerides and cholesterol.
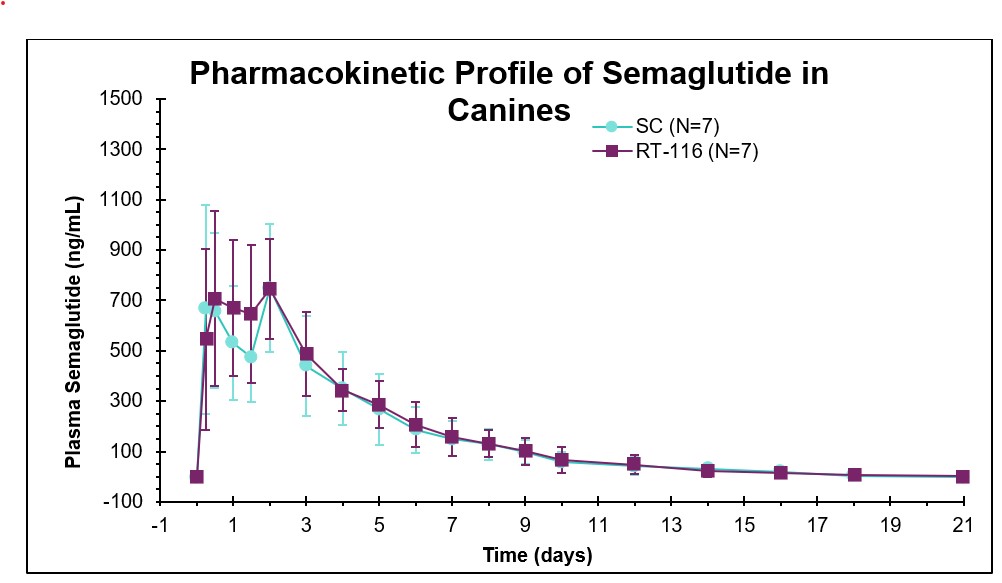
| Route | Cmax (ng/mL) |
Tmax (days) |
Tlast (days) |
AUClast (ng/mL*day) |
| Oral (RT-116) | 941 ± 90 | 1.3 ± 0.3 | 17 ± 2 | 3630 ± 222 |
| SC | 948 ± 120 | 1.3 ± 0.3 | 17 ± 1 | 3390 ± 402 |
| All data is mean ± standard error. | ||||
Near-Term Milestone Expectations:
- Initiation of Phase 1 clinical trial of RT-114 containing a GLP-1/GLP-2 dual agonist for the treatment of obesity expected in 2025.
About Rani Therapeutics
Rani Therapeutics is a clinical-stage biotherapeutics company focused on advancing technologies to enable the development of orally administered biologics and drugs. Rani has developed the RaniPill® capsule, which is a novel, proprietary and patented platform technology, intended to replace subcutaneous injection or intravenous infusion of biologics and drugs with oral dosing. Rani has successfully conducted several preclinical and clinical studies to evaluate safety, tolerability and bioavailability using RaniPill® capsule technology. For more information, visit ranitherapeutics.com.
Forward-Looking Statements
Statements contained in this press release regarding matters that are not historical facts are “forward-looking statements” within the meaning of the Private Securities Litigation Reform Act of 1995. Such forward-looking statements include statements regarding, among other things, the expected initiation of a Phase 1 trial of RT-114 in 2025, the potential of the RaniPill® platform to enable oral delivery of multiple obesity treatments and validation of such potential through preclinical data, the target product profile of semaglutide delivered via the RaniPill® capsule, the novelty, convenience and attractiveness of a once-weekly oral semaglutide treatment for patients, Rani’s confidence to continue building an obesity portfolio, the potential of a once-weekly oral version of semaglutide in the RaniPill® capsule to deliver the same benefits as subcutaneous delivery and to impact patients’ lives, the sufficiency of Rani’s cash reserves, and future financial performance. Because such statements are subject to risks and uncertainties, actual results may differ materially from those expressed or implied by such forward-looking statements. Words such as “believe,” “would,” “potential,” “expect,” “continue” and similar expressions are intended to identify forward-looking statements. These forward-looking statements are based upon Rani’s current expectations and involve assumptions that may never materialize or may prove to be incorrect. Actual results could differ materially from those anticipated in such forward-looking statements as a result of various risks and uncertainties, which include, without limitation, risks and uncertainties associated with Rani’s business in general and the other risks described in Rani’s filings with the Securities and Exchange Commission, including Rani’s annual report on Form 10-K for the year ended December 31, 2023, and subsequent filings and reports by Rani. All forward-looking statements contained in this press release speak only as of the date on which they were made and are based on management’s assumptions and estimates as of such date. Rani undertakes no obligation to update such statements to reflect events that occur or circumstances that exist after the date on which they were made, except as required by law.
Investor Contact:
[email protected]
Media Contact:
[email protected]
Photos accompanying this announcement are available at:
https://www.globenewswire.com/NewsRoom/AttachmentNg/b1fecb62-a30c-4b59-9234-245cebe4e59e
https://www.globenewswire.com/NewsRoom/AttachmentNg/d32ef495-95db-41e6-8429-a597576d5ed1
An attachment accompanying this announcement is available at:
https://www.globenewswire.com/NewsRoom/AttachmentNg/61c2f390-a3c5-45ab-901e-37f49f6ab4f2





